Demystifying Boyle’s Law: Understanding the Relationship Between Pressure and Volume
Boyle’s Law, a fundamental principle in the field of physics and gas chemistry, elucidates the relationship between the pressure and volume of a gas at constant temperature. This law, formulated by Irish scientist Robert Boyle in the 17th century, is a cornerstone of our understanding of gas behavior. In this article, we will explore Boyle’s Law, its origins, its practical applications, and its relevance in the modern scientific and industrial landscape.
I. The Origin of Boyle’s Law
- Robert Boyle’s Contribution: Robert Boyle, a polymath and one of the founders of the Royal Society, was intrigued by the behavior of gases. In 1662, he published “The Sceptical Chymist,” which questioned prevailing alchemical theories and laid the groundwork for modern chemistry. Boyle’s most famous contribution, however, was the formulation of Boyle’s Law, which he described in his work “New Experiments Physico-Mechanical, Touching the Spring of the Air, and its Effects” in 1662.
- Boyle’s Experiment: Boyle conducted experiments using a J-shaped glass tube (now known as a Boyle’s tube) partially filled with mercury and a trapped volume of gas. By altering the volume of the gas and recording the corresponding pressure, he discovered an inverse relationship—when the volume increased, the pressure decreased, and vice versa. This pivotal experiment led to the formulation of Boyle’s Law.
II. The Statement of Boyle’s Law
Boyle’s Law is often stated as follows:
“For a given mass of gas at constant temperature, the pressure of the gas is inversely proportional to its volume.”
Mathematically, this relationship can be expressed as:
P1 * V1 = P2 * V2
Where:
- P1 and P2 are the initial and final pressures, respectively.
- V1 and V2 are the initial and final volumes, respectively.
This equation underscores the critical principle of the law: as the volume of a gas increases, its pressure decreases, and vice versa, provided that temperature remains constant.
III. The Concept of an Inverse Relationship
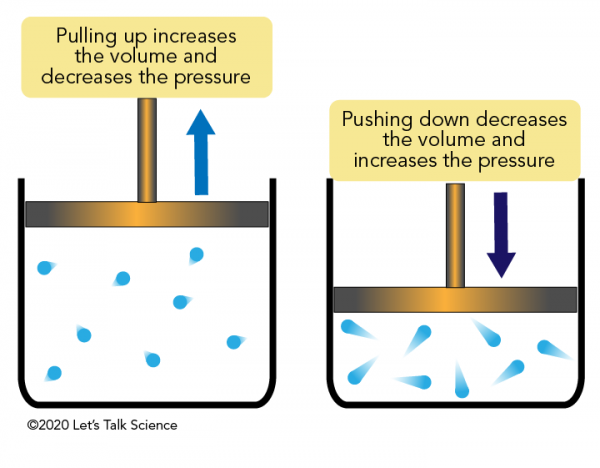
Understanding the inverse relationship at the heart of Boyle’s Law is essential. When the volume of a gas is reduced (for instance, by compressing it into a smaller space), the gas particles are closer together, and their collisions with the container walls occur more frequently and with greater force. Consequently, the pressure increases. Conversely, if the volume is increased (by expanding the gas into a larger space), the gas particles are more dispersed, leading to fewer and less forceful collisions with the container walls, resulting in lower pressure.
IV. Practical Applications of Boyle’s Law
Boyle’s Law finds practical applications in various fields, including:
- Aerospace Engineering: Understanding how pressure and volume affect the behavior of gases is critical for designing spacecraft and life support systems where pressure changes can occur.
- Chemical Industry: Industries that produce, store, or transport gases use Boyle’s Law principles to optimize processes, such as liquefying gases for storage and transportation.
- Medicine: In the medical field, Boyle’s Law is relevant to the behavior of gases in the respiratory system, especially during artificial ventilation and diving.
- Environmental Science: The behavior of gases in Earth’s atmosphere, particularly in relation to changes in pressure and volume with altitude, is a key aspect of environmental science.
V. The Impact of Temperature and the Combined Gas Law
While Boyle’s Law focuses on the relationship between pressure and volume at constant temperature, it is essential to acknowledge that temperature also plays a significant role in gas behavior. When temperature changes, the pressure and volume of a gas can vary accordingly. In this context, Boyle’s Law is often integrated with Charles’s Law and Gay-Lussac’s Law to form the Combined Gas Law.
The Combined Gas Law is expressed as:
(P1 * V1) / T1 = (P2 * V2) / T2
This law combines the relationships between pressure and volume (Boyle’s Law) and volume and temperature (Charles’s Law) into one comprehensive equation.
VI. Practical Demonstrations of Boyle’s Law
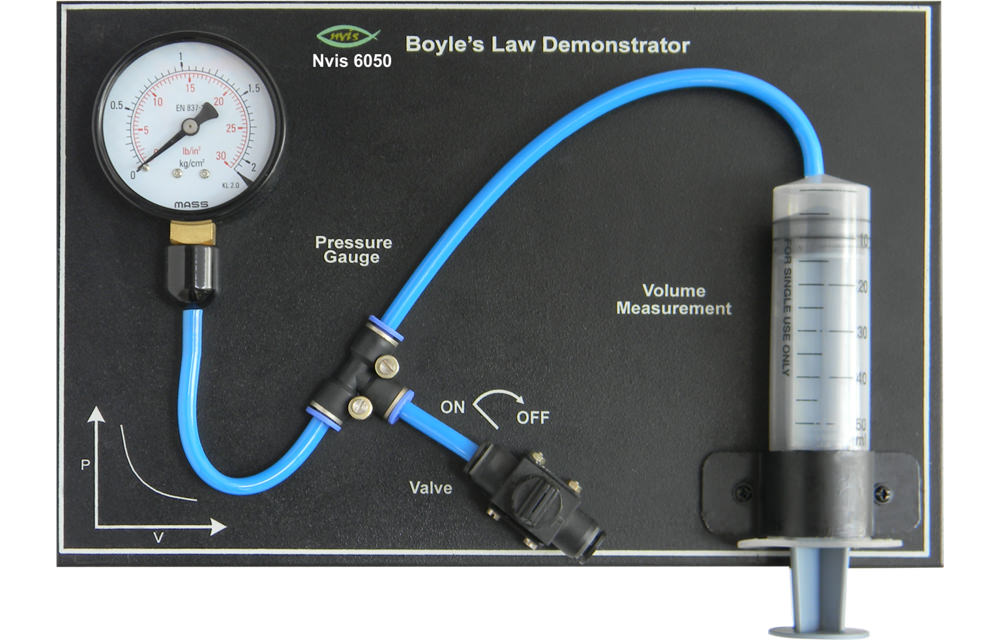
Demonstrations of Boyle’s Law can be performed using simple experiments. One common approach involves a syringe and a pressure sensor. By manipulating the volume of gas inside the syringe and measuring the pressure, the inverse relationship predicted by Boyle’s Law can be observed.
VII. The Limitations of Boyle’s Law
Boyle’s Law, while fundamental, has limitations. It is most accurate when dealing with ideal gases under ideal conditions—low pressure and high temperature. Real gases, which have interactions between particles, do not always adhere perfectly to Boyle’s Law, particularly at high pressures and low temperatures.
VIII. Conclusion
Boyle’s Law, formulated by Robert Boyle in the 17th century, provides a foundational understanding of the inverse relationship between the pressure and volume of gases at constant temperature. This law has found widespread applications in fields ranging from aerospace engineering to the chemical industry. It underscores the fundamental nature of gas behavior, which is crucial in scientific research, industrial processes, and everyday life. While Boyle’s Law has its limitations, it remains a cornerstone of our understanding of the behavior of gases and continues to shape scientific and technological advancements in the modern world.